Easy to use
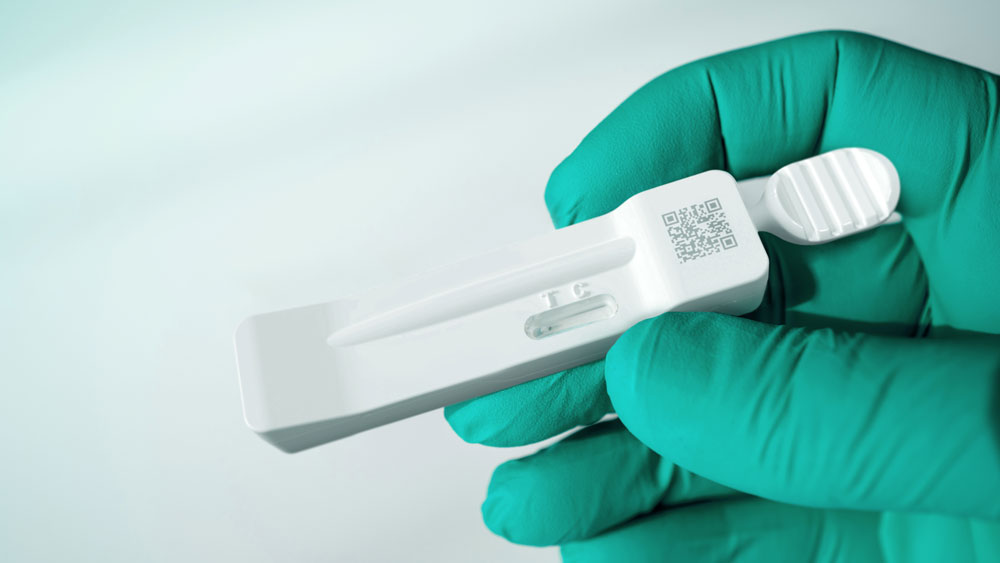
The Gen 3.1 product offers all of the benefits of Gen 3 with the added feature of being hermetically sealed once the swab is secured in the cassette. This makes the Gen 3.1 design perfect when testing for biomarkers of highly hazardous viruses or disease.
Enhanced
The Gen 3.1 product is very similar in size to the Gen 3 product and as such retains all the packaging and logistic benefits. The minimal packaging requirement means that even when providing hazardous waste material disposal packaging the finished product is still up to 60% smaller than that of a traditional rapid antigen test kit.
Minimal costs, maximum safety
The next-generation design of Gen 3.1 comes at a minimal additional manufacturing cost, because we believe you shouldn’t pay over the odds for the enhanced control and containment of severe and sometimes fatal infections.
Gen 3.1 takes the next step in lateral flow testing platforms by significantly increasing contamination and contagion control. A unique locking swab feature prevents leakage during the undertaking of a test, while a transparent window protects the sample after the test.
Thanks to the innovative design, this enhanced-safety product variant is produced at only a small extra cost compared to Gen 3 – a crucial factor for mass self-testing scenarios where there is a greater need for virus containment and control. Applications include the testing for MRSA, strep A, RSV, influenza, and SARS-COV-2. For tests involving a blood specimen, please see Gen 3.3b
Learn more about Gen 3.1
Reach out for more information on how Gen 3.1 can be utilised in real mass testing scenarios, along with our various avenues to market for global organisations and users.